The Hidden Dangers in Buspirone Tablets: How Impurities Could Lead to Severe Health Risks—Even Death
The Hidden Dangers in Buspirone Tablets: How Impurities Could Lead to Severe Health Risks—Even Death
Blog Article
Major Side Effects of Impurities in Buspirone Tablets
Buspirone is widely prescribed for managing anxiety disorders, but when this medication contains uncontrolled impurities, it can become dangerous rather than therapeutic. Impurities in Buspirone tablets may arise from raw materials, manufacturing processes, degradation over time, or poor storage conditions—and their impact can be severe.
1. Neurotoxic Reactions
Unidentified impurities can affect the central nervous system, intensifying symptoms such as:
- Confusion
- Hallucinations
- Seizures
- Sudden mood swings or psychosis
These effects can be mistaken for worsening anxiety but may stem from chemical contaminants.
2. Hepatotoxicity (Liver Damage)
The liver is responsible for metabolizing Buspirone. Impurities can burden or damage hepatic function, leading to:
- Elevated liver enzymes
- Jaundice (yellowing of skin or eyes)
- Fatigue and nausea
- In extreme cases, acute liver failure
3. Cardiovascular Complications
Toxic impurities may disrupt cardiovascular function, potentially causing:
- Irregular heart rhythms
- Elevated blood pressure
- Chest pain
- Increased risk of cardiac arrest
4. Allergic or Hypersensitivity Reactions
Some impurities may act as allergens or irritants, triggering:
- Skin rashes
- Swelling of the face, tongue, or throat
- Difficulty breathing
- Anaphylaxis (a life-threatening allergic response)
5. Mutagenic or Carcinogenic Risks
Certain genotoxic impurities can interfere with DNA, increasing the risk of:
- Long-term genetic mutations
- Development of cancers with prolonged exposure
6. Drug Interaction and Reduced Efficacy
Impurities can alter the drug’s pharmacokinetics, making Buspirone:
- Less effective in treating anxiety
- More likely to interact harmfully with other medications
What Are Impurities in Pharmaceuticals
Impurities are unintended substances that may be present in active pharmaceutical ingredients (APIs) or finished drug products. These can arise from:
- Raw materials
- Synthesis processes
- Degradation over time
- Storage conditions
Identifying and managing these impurities ensures:
- Patient safety
- Regulatory compliance
Why Impurity Profiling Matters
Impurity profiling refers to the detection, identification, and analysis of unwanted substances. This process is essential for several reasons:
- Safety First: Some impurities can be toxic. Profiling helps identify and minimize these risks.
- Regulatory Approval: Authorities like the FDA and EMA require detailed impurity profiles for drug approval.
- Quality Control: Monitoring ensures consistency and reliability across production batches.
OMCHEM LABS: A Global Leader in Impurity Profiling
Founded with a vision to elevate pharmaceutical standards, OMCHEM LABS has become a trusted name in impurity profiling. Backed by state-of-the-art R&D facilities and experienced scientists, the company delivers:
- Technical excellence
- Regulatory insight
- Global support
They specialize in:
- Custom Impurity Synthesis
– Tailored impurity development for antibiotics, steroids, and both chiral and achiral drugs.
- Custom Impurity Synthesis
- Certified Reference Standards (CRS)
– High-purity benchmarks for analytical testing with a vast impurity catalog and growing chemical database.
- Certified Reference Standards (CRS)
Analytical Expertise at Its Best
OMCHEM LABS provides advanced analytical capabilities to ensure precision in impurity analysis, including:
- Method Development & Validation
– Creating and validating techniques for trace-level impurity detection.
- Method Development & Validation
- Stability Testing
– Studying how drug compounds degrade and the impurities they form over time
- Stability Testing
- Structure Elucidation
– Using advanced instrumentation to determine the molecular structure of unknown impurities.
- Structure Elucidation
These services help companies maintain global compliance and ensure safe, high-quality pharmaceutical products.
Supporting Regulatory Success
Navigating global pharmaceutical regulations can be complex. OMCHEM LABS supports clients with:
- Regulatory documentation & dossier preparation
- Submissions for Drug Master Files (DMFs) and Abbreviated New Drug Applications (ANDAs)
- Expertise to meet international regulatory expectations
A Global Partner You Can Trust
OMCHEM LABS holds various international certifications and has been audited by leading agencies, reflecting their dedication to:
- Quality
- Compliance
- Global pharmaceutical standards
Buspirone impurities can form during the synthesis of the active pharmaceutical ingredient (API) and formulation processes due to side reactions, degradation of the compound under specific environmental or processing conditions, or contamination from solvents and reagents. These impurities may result from unstable intermediates, over-reaction, or deviations in reaction parameters during synthesis.

Buspirone Impurity A
CAS No.: 20980-22-7

Buspirone Impurity B
CAS No.: 81461-73-6

Buspirone Impurity C
CAS No.: 257877-45-5

Buspirone Impurity E
CAS No.: 257877-43-3

Buspirone Impurity F
CAS No.: 2512210-24-9

Buspirone Impurity G
CAS No.: 84746-24-7

Buspirone Impurity I
CAS No.: 2725354-99-2
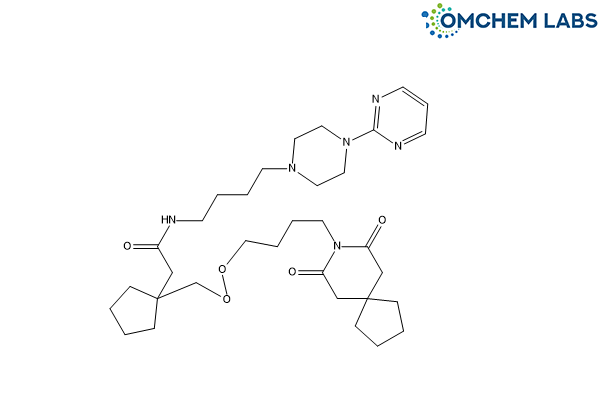
Buspirone Impurity J
CAS No.: 2726492-72-2

Buspirone Impurity K
CAS No.: 1075-89-4

Buspirone Impurity L
CAS No.: 21098-11-3

Buspirone Impurity M
CAS No.: 80827-62-9

Buspirone Impurity N
CAS No.: 257877-44-4

Buspirone Hydrochloride
CAS No.: 33386-08-2
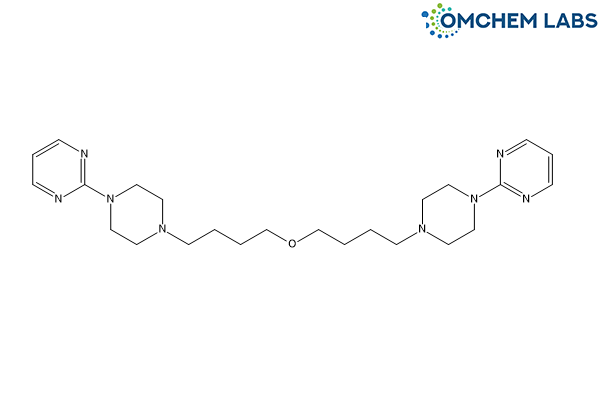
Buspirone Impurity D (Free Base)
CAS No.: 2724726-67-2

Buspirone Impurity D (HCL)
CAS No.: N/A

Buspirone N-Oxide
CAS No.: 220747-81-9
Final Thoughts
Impurity profiling is not just a regulatory checkbox—it’s a vital part of pharmaceutical safety, effectiveness, and trust. With:
- Unmatched expertise
- Global presence
- Relentless focus on quality
OMCHEM LABS continues to lead the way in impurity profiling and reference standard development for the pharmaceutical industry. Report this page
